Integrated Analysis of Gut Metabolome, Microbiome, and Exfoliome Data in an Equine Model of Intestinal Injury
Abstract
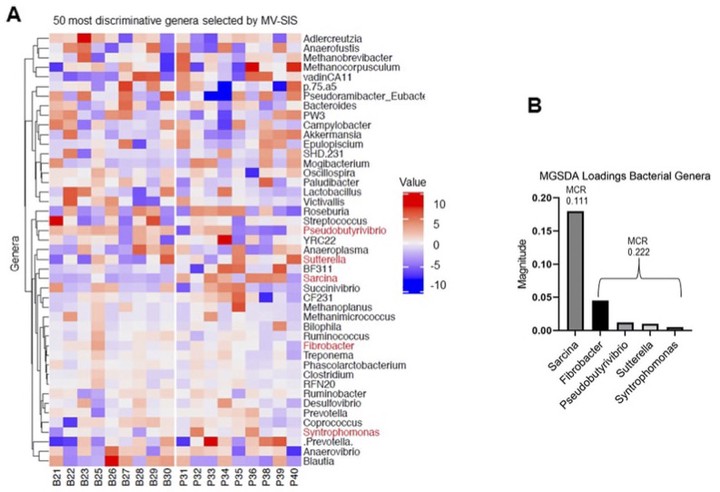
The equine gastrointestinal (GI) microbiome has been described in the context of various diseases. The observed changes, however, have not been linked to host function and therefore it remains unclear how specifc changes in the microbiome alter cellular and molecular pathways within the GI tract. Further, non-invasive techniques to examine the host gene expression profle of the GI mucosa have been described in horses but not evaluated in response to interventions. Therefore, the objectives of our study were to profile gene expression and metabolomic changes in an equine model of non-steroidal anti-infammatory drug (NSAID)-induced intestinal infammation and apply computational data integration methods to examine host-microbiota interactions. Twenty horses were randomly assigned to 1 of 2 groups, control (placebo paste) or NSAID (phenylbutazone 4.4 mg/kg orally once daily for 9 days). Fecal samples were collected on days 0 and 10 and analyzed with respect to microbiota (16S rDNA gene sequencing), metabolomic (untargeted metabolites), and host exfoliated cell transcriptomic (exfoliome) changes. Data were analyzed and integrated using a variety of computational techniques, and underlying regulatory mechanisms were inferred from features that were commonly identifed by all computational approaches. Phenylbutazone induced alterations in the microbiota, metabolome, and host transcriptome. Data integration identifed correlation of specifc bacterial genera with expression of several genes and metabolites that were linked to oxidative stress. Concomitant microbiota and metabolite changes resulted in the initiation of endoplasmic reticulum stress and unfolded protein response within the intestinal mucosa. Results of integrative analysis identifed an important role for oxidative stress, and subsequent cell signaling responses, in a large animal model of GI infammation. The computational approaches for combining noninvasive platforms for unbiased assessment of host GI responses (e.g., exfoliomics) with metabolomic and microbiota changes have broad application for the feld of gastroenterology.